
What does this have to do with splitting sugars? I had a friend who, during trips up the coast of California would always send this postcard. The back was simply inscribed: "we are splitting atoms in Buellton".
Living in Los Angeles as college students many years ago meant the long Friday night drive to visit high school friends in Berkeley, Santa Cruz, or San Luis Obispo. As today, we searched for the quirky restaurants where the owner was behind the stove or behind the bar (Hungry? Eat at Whizzins!). One of the staples was Andersen's, the temple of split pea soup.
Anton Andersen, founder of the said restaurant, was a renowned chef who had grow tired of the incessant pressure of New York hotel kitchens and decided to move west. He and his wife bought a spot of land and a small building in Buellton, California. With their last savings they christened a new electric stove and opened their doors to the world. The business? Logically, it was called Andersen's Electric Cafe (1).
Andersen, understanding the art of the great kitchens, donned the apron of an artisan, and began preparing split pea soup from scratch. Truckers and highway travelers of the 1930's began to tell friends of the hospitality and great pea soup in Buellton. Anton began by buying 50 pounds of peas a month, soon it would be a ton. By the time struggling physics students were heading north on route 101 to enjoy a bowl of soup, the third generation of owners were cooking 50 tons of split peas into soup a year on the premises. There was still the mix of art, artisans, and whimsy at the restaurant. Besides the kiddie park and wild animal park, the owners became enamored with parrots, and straightforwardly built an aviary adjacent to the kitchens.
Unfortunately, the vagaries of civil aviation ended the fancies of the owner and his family in 1980. For the trustees of the estate, there was only one 'reasonable' path, and that was to maximize the value of the Andersen's brand. Art and artisans were forgotten in favor of the assembly line. The classic recipe was sold, analyzed, dissected, scaled up, made 'shelf-stable' all under the skilled hand of "The leading North American co-pack manufacturer of aseptically manufactured products" (Only American Industry can think up such mission statements*). Food became 'product'. Cooking became easier if all one had to do was assemble fewer and fewer 'products' to make a meal. Eating? There was time to be saved there too...for what, I do not know.
In splitting sugar: Know the art; practice as an artisan; eat as human beings.
Invert sugar syrup
Yeast is a thrifty organism. It likes to start life converting glucose to carbon dioxide and alcohol. However, yeast was never intended to digest sucrose, or regular table sugar. For this task, yeast comes equipped with at least 10 enzymes such as invertase that can break down complex sugars and carbohydrates into what yeast likes best: glucose and fructose.
To give new yeast a good start, I like to add a bit of invert syrup to the hydration water. This used to come from a wonderful sugar syrup sold in Miami as 'melao', produced in Puerto Rico. Now that we live in Puerto Rico, we cannot find melao anywhere so duplicating it became the goal.
What is the art? Table sugar is a di-saccharide, made up of glucose and fructose. The two simple sugars are held together by a stretchy oxygen atom linking the two structures (it is the dizzy atom at the center):
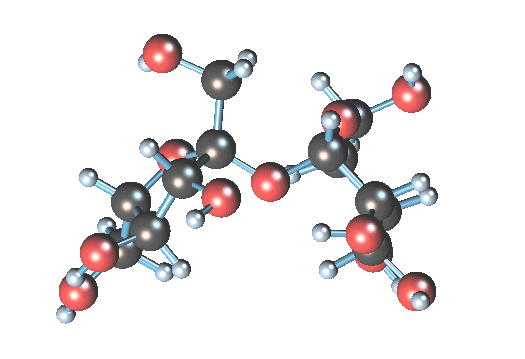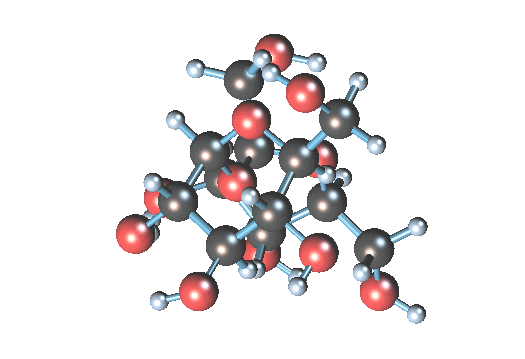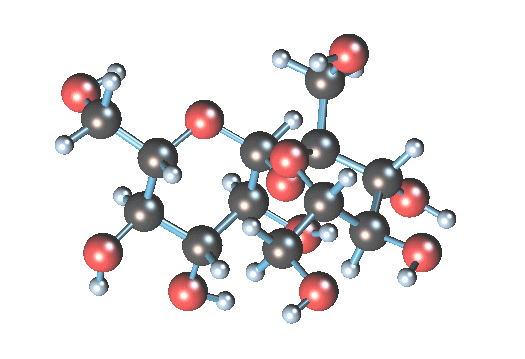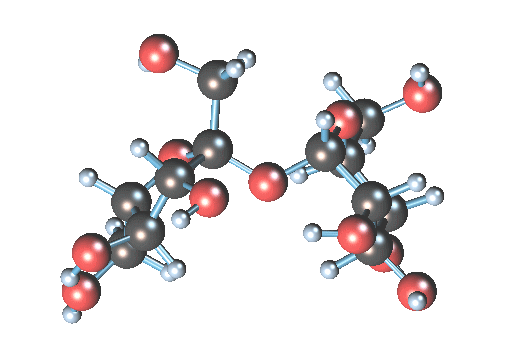 In water, the H2O can convince the stressed oxygen atom to let go. Water gives up one of its hydrogen atoms to the sucrose oxygen, making an hydroxyl group (-OH) and leaving an extra (HO-). The sucrose molecule breaks apart into glucose (the 6-atom ring) and fructose (the pentagonal ring). The result is invert syrup.
In water, the H2O can convince the stressed oxygen atom to let go. Water gives up one of its hydrogen atoms to the sucrose oxygen, making an hydroxyl group (-OH) and leaving an extra (HO-). The sucrose molecule breaks apart into glucose (the 6-atom ring) and fructose (the pentagonal ring). The result is invert syrup.Simplistically, you could just mix sugar and water and wait, and wait, and wait, since the above hydrolysis reaction is somewhat slow. You could heat the water to better dissolve the sucrose, and it would speed the reaction so a reasonable amount of glucose would be ready in several days. However, the problem with hot water hydrolysis is that undesirable by-products sometimes are formed by further breakdown of the sugar. To really make the reaction move, you need to add a tiny bit of acid. The acid is a catalyst that accelerates the process by 10-100 times. The (very free) hydrogen radical (H+) in acid is a catalyst, creating many hydroxyls that can then happily split the sucrose molecule, without being itself consumed.
To make a very thick, honey like syrup.
If your 100 parts of water are 100cc and your sugar is 300 grams, you will make about 300cc of syrup with a total of 1200 calories (4 calories per cc).
- 100 parts of water
- 300 parts of raw brown sugar
- 0.5 parts of acetic acid (If you are using 5% white vinegar, use 10 parts of vinegar)


The 300 grams of sugar on the left probably cost someone a good deal of sweat in the Dominican Republic.
The container on the right is filtered water, not sweat.
Dissolve the sugar in water in a non-reactive pan. It will not really dissolve but appear as a sandy mix. Slowly raise the temperature of the solution to 100C. When the slightest, wispy bubbles begin to swirl on the surface, add the vinegar At this concentration, a slight boil will begin at about 104-105C.


Finally the wispy veil of tiny bubbles on the surface at 104C.
5cc of 5% white vinegar sets off the (mainly quiet) reaction.
Stop the boil and lower the temperature to 80C for a period of an hour. This will give more than sufficient time for the acid to work. The lower temperature precludes caramelization and off flavors. Since the quantity is so small, there is no 'vinegar' flavor left at the end of the acid hyrolysis.

Transformation.
0.87 of a mole of sucrose meets 5.56 moles of water
under the guidance of 0.008 mole of acetic acid
Example
Puerto Rico has the most flavorful citrus fruit that I have experienced. This is in spite of the fruit being scarred, scratched, scraped, and generally touched with black sooty mold. Grapefruit, orange, sweet lemon and limón trees are left untended in backyards throughout the island, making them quite natural, but also quite beat up. Sometimes you can just ask to pick fruit and share the harvest with the host. When not doing this, we buy hand picked from our local organic farmer, SIEMBRA TRES VIDAS. If you juice the grapefruit, you miss the wonder full oils in the skin. Here is a neat way to get all the flavor while using about 2-3cc of the newly made invert syrup.
- Squeeze 1 grapefruit (the amount of juice in one Puerto Rican grapefruit is deceiving. Our building's bylaws advise against juicing more than one to prevent damage to apartments below.)
- Take half the rind, cut into coarse chunks, and pound with a meat tenderizer.
- Add the crushed rind into a large cocktail shaker
- Add the juice
- Add 2-3 cc of invert syrup
- Add 2-3 ice cubes.





No comments:
Post a Comment